Aluminum And Oxygen Ionic Compound
Aluminium oxide formula is given here to help pupil hands learn and call back the formula during course tests or even in semester exams. Aluminium oxide is a chemical chemical compound of oxygen and aluminium and occurs naturally in various minerals such as corundum, bauxite, etc. This compound is by and large amphoteric in nature and basically has chemical, industrial and commercial applications. Let us await at the formula below.
Aluminium Oxide Chemical Formula
The formula of aluminium oxide is given as Al2O3. How is this formula generated? First of all, we have to consider that aluminium is a metallic and Oxygen is a non-metal making it an ionic compound. There is some charge in the aluminium and oxygen ions. Aluminium has a +iii charge whereas oxygen has -2 accuse. On applying the criss-cantankerous method we get the formula of aluminium oxide. Unremarkably, aluminium loses three electrons while oxygen gains two electrons in the chemic process and this is mainly done to achieve stability.
| Formula | AltwoOthree |
| Molar Mass | 101.960 thousand·mol−i |
| Density | 3.987g/cm3 |
| Melting Bespeak | two,072 °C |
| Humid Point | 2,977 °C |
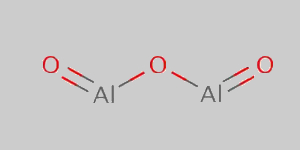
Aluminium Oxide Structural Formula
Below is the diagram of the aluminium oxide structural formula.
Students tin can cheque out the different formulas of important chemical compounds here at BYJU'S.
Aluminum And Oxygen Ionic Compound,
Source: https://byjus.com/aluminium-oxide-formula/
Posted by: overturffrect1967.blogspot.com


0 Response to "Aluminum And Oxygen Ionic Compound"
Post a Comment